.png)
New GLP AAV-ITR Sequencing for FDA Submissions
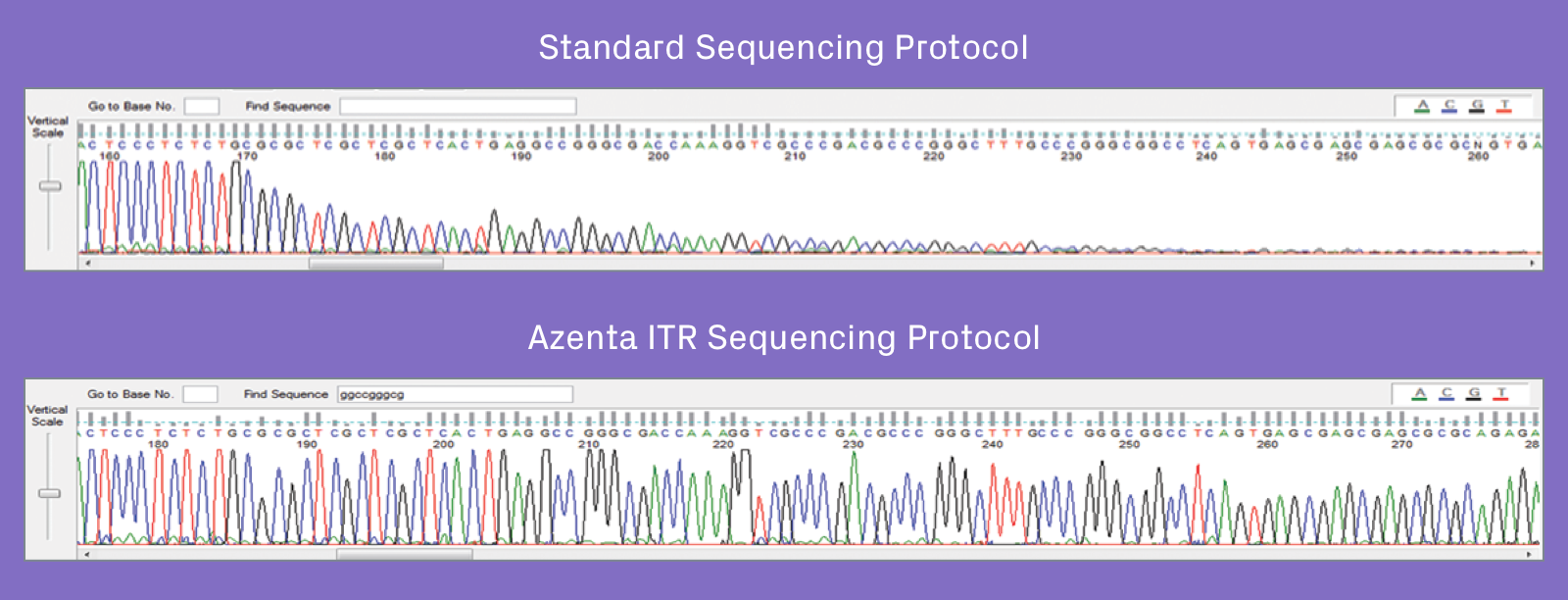
Azenta's proprietary AAV-ITR Sanger sequencing protocol is now available as a GLP-compliant (21 CFR part 58) workflow to support FDA IND, NDA, and BLA submissions. Our unique protocol sequences through difficult inverted terminal repeat (ITR) regions of adeno-associated virus (AAV), prevents abrupt reduction in the sequencing signal at the start of the ITR hairpin, and reads through the full length of the ITR region.
Check out our tech note for more information on our proprietary AAV-ITR Sanger sequencing protocol. This novel technology currently enables GLP-compliant whole product sequencing.
Contact us to learn more.
Service Highlights
![]()
GLP-compliant whole AAV plasmid sequencing for confirmation and genetic stability studies
![]()
Increased read lengths and improved data quality allow for early detection of point mutations
![]()
Proprietary GLP-compliant AAV-ITR method provides greater ITR sequencing coverage compared to standard protocols
![]()
Dedicated Study Director for proactive, transparent communication throughout your entire project
![]()
Final report includes a description of methods, list of SOPs, detailed analysis, signed GLP compliance statement, and QA inspections
Features & Benefits
![]()
Complete solutions pipeline from nucleic acid extraction to data analysis
![]()
Assay development expertise excelling in assay optimization and handling difficult templates
![]()
Industry-leading turnaround with options for expedited assay development and sequencing
![]()
State-of-the-art GLP laboratory with Quality Assurance Unit oversight ensures your project will meet FDA guidelines