AAV Plasmid Prep Tech Note
An Efficient and High-Fidelity Approach to AAV Plasmid Preparation
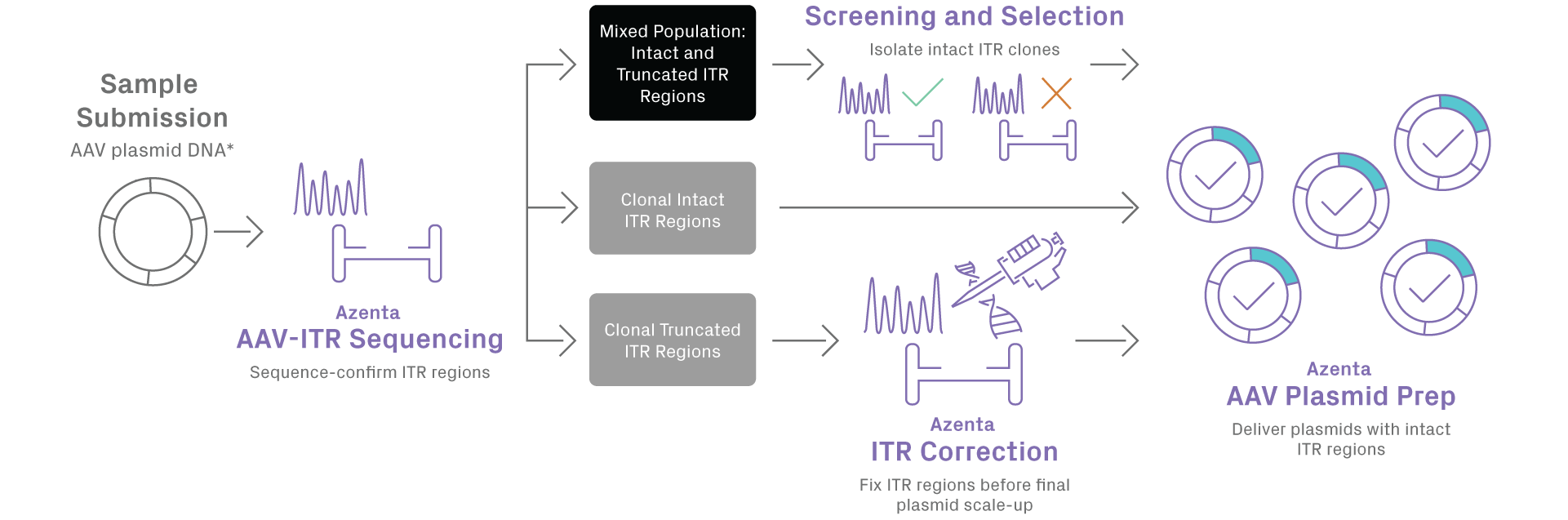
Adeno-associated virus (AAV) plasmid preparation is essential for gene therapy and AAV vector production, yet maintaining inverted terminal repeat (ITR) integrity remains a persistent challenge. During bacterial propagation, 5–15% of AAV plasmid preparations develop deletions that reduce viral packaging efficiency, and standard quality control methods, such as restriction digests and Sanger sequencing, cannot accurately detect ITR mutations due to their complex secondary structure.
This technote highlights GENEWIZ from Azenta Life Sciences’ high-fidelity AAV plasmid preparation workflow, designed to overcome ITR instability. Using proprietary bacterial strains that stabilize ITR sequences and specialized AAV-ITR sequencing for single-nucleotide mutation detection, our platform achieves a 98% success rate in producing plasmids with fully intact ITRs. Researchers gain reliable, reproducible AAV constructs for gene therapy, viral vector design, and preclinical development.
Proprietary AAV Plasmid Synthesis Workflow

Download Tech Note
Related Synthesis Services
Custom AAV plasmid design and synthesis optimized for packaging, expression, and ITR fidelity.
Reliable AAV plasmid DNA preparation with high yield, purity, and ITR protection.
AAV-ITR Sanger sequencing for ITR integrity validation and vector QC.